Medical mask Type IIR Kolmi Softex

Medical device : Class I - Type of medical mask : Type IIR - Earloops - Without option(s)
Ref. M35-SANS OPTIONS
-
Dental -
Medical -
Industry -
Hygiene
FEATURES AND BENEFITS
TECHNICAL SPECIFICATIONS
PRODUCT INFORMATION
CERTIFICATIONS & STANDARDS
DOCUMENTS
Features and benefits
- Protects the environment from droplets emitted by the wearer while ensuring protection against splashes of biological fluids
- Effective against bacteria, particles, and viruses (tested for BFE, PFE, & VFE)
- Kolmi® masks feature the exclusive Origami fold and Smile welding for enhanced comfort and a perfect fit to the wearer's face
- For maximum comfort, the inner layer is designed with Softex technology, which minimizes facial irritation and does not wear out during prolonged use, even in contact with a beard
- Clinically tested and recognized by AFPRAL, wearing the Kolmi® Softex Type IIR medical mask reduces symptoms of seasonal allergies (rhinitis, nasal congestion, conjunctivitis, cough, asthma, sneezing, headaches). It significantly improves the quality of life for individuals allergic to pollen (pilot clinical study Alyatec 2022/ AFPRAL = French Association for the Prevention of Allergies)
- Hypoallergenic
Technical specifications
- Type of fastener : Earloops
-
Colors :
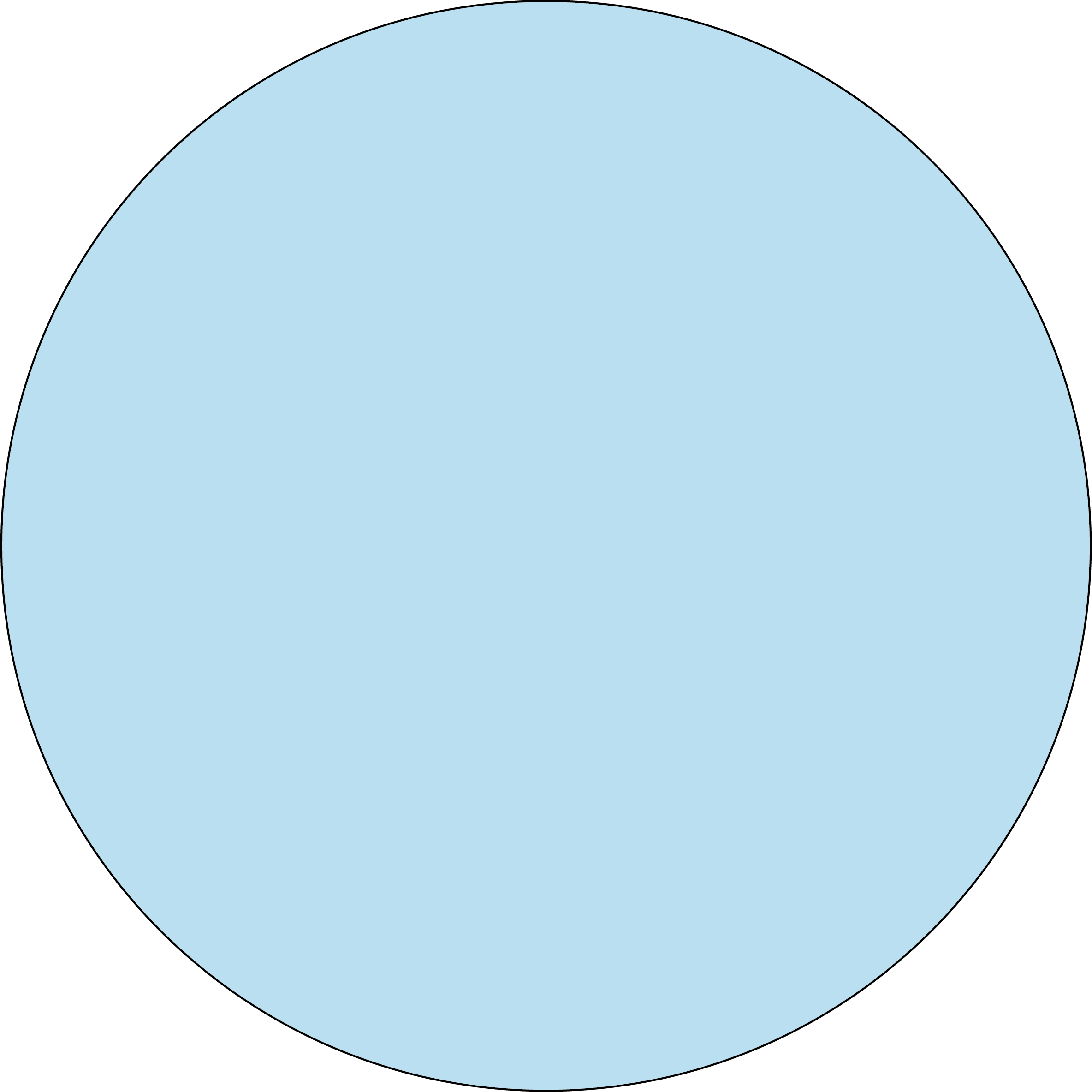 Blue
Blue
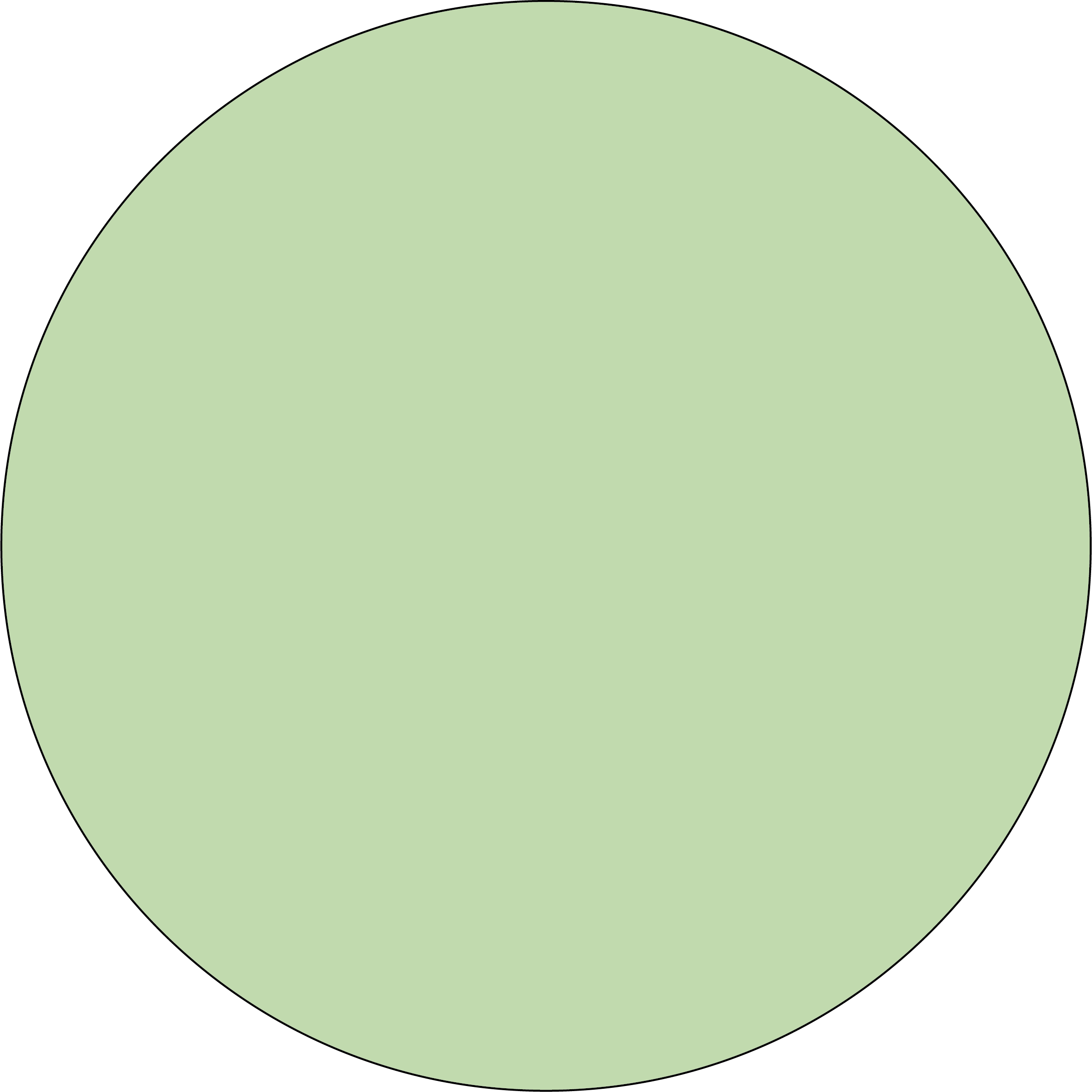 Green
Green
 Pink
Pink
- Size / Dimensions : 175 x 90 mm
- Option : Without option(s)
- Made in : France
Product information
Product features
| Article | Size | Color |
|---|---|---|
| M35111-30 | 175 x 90 mm |  Blue Blue |
| M35211-30 | 175 x 90 mm |  Green Green |
| M35511-30 | 175 x 90 mm |  Pink Pink |
Packaging Features
| Inner case | Packaging |
|---|---|
| Box | 16 x 50 Units |
| Box | 16 x 50 Units |
| Box | 16 x 50 Units |
Certifications & standards
- ISO 9001 Manufacturing site
- ISO 13485 Manufacturing site
- Compliant to EU Regulation 2017/745 on Medical Devices
- ISO 10993-5
- ISO 10993-10
- Assessment of microbial contamination according to ISO 11737 : 2018 + A1 : 2021