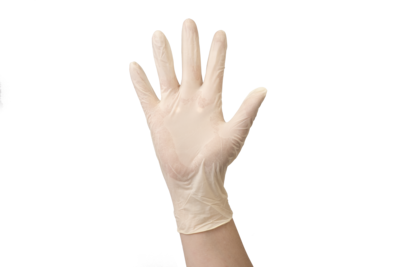
Glove SafeTouch® Connect

Medical device : Class I - Personal Protective Equipment : Cat. III - Latex - Not powder
Ref. 1124N*
-
Dental -
Medical -
Industry -
Hygiene
FEATURES AND BENEFITS
TECHNICAL SPECIFICATIONS
PRODUCT INFORMATION
CERTIFICATIONS & STANDARDS
DOCUMENTS
Features and benefits
- Great elasticity.
- Good dexterity.
- Its textured appearance provides excellent grip on objects and facilitates their handling.
- Waterproof and airtight glove.
- Latex allows for excellent touch sensitivity.
- Contraindication: latex is a natural material that contains allergenic proteins.
Technical specifications
- Material : Latex
- Colors : Natural
- Size / Dimensions : XS / S / M / L / XL
- Integrated lotion : non
-
Texture :
Fully textured
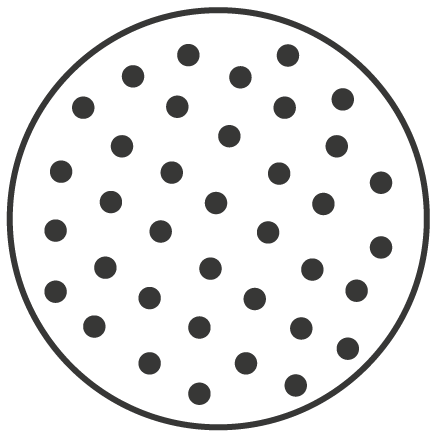
-
Powder :
Not powder

- Shape : Ambidextrous
- Glove EN 455-2 length : 240,00 mm
- Made in : Malaysia
Product information
Product features
| Article | Size | Color |
|---|---|---|
| 1124N-A | XS |  Natural Natural |
| 1124N-B | S |  Natural Natural |
| 1124N-C | M |  Natural Natural |
| 1124N-D | L |  Natural Natural |
| 1124N-E | XL |  Natural Natural |
Packaging Features
| Inner case | Packaging |
|---|---|
| Dispenser box | 10 x 100 Units |
| Dispenser box | 10 x 100 Units |
| Dispenser box | 10 x 100 Units |
| Dispenser box | 10 x 100 Units |
| Dispenser box | 10 x 100 Units |
Certifications & standards
- ISO 9001 Manufacturing site
- ISO 13485 Manufacturing site
- Compliant to EU Regulation 2017/745 on Medical Devices
- EN 455-3 / Biological Evaluation
- EN 455-4 / Shelf life
- EN 455-1 / Hole protection
- ISO 10993 / Biocompatibility
- EN 455-2 / Physical Properties Tests
- Compliant to EU Regulation 2016/425 on Personal Protective Equipment
- Chemical protection / EN ISO 374-1 Type B, EN ISO 374-2, EN ISO 374-4, EN 16523-1 - TYPE B MST
- Microbial protection / EN ISO 374-5, EN ISO 374-2
- Virus protection / EN ISO 374-5, ISO 16604
- EN ISO 21420
- EU Regulation 1935/2004
- French decree of 05/08/2020
- EU Regulation 10/2011
- Order of 09/11/1994